The TPD of EU countries and the MHRA registration fee of the UK. The Tobacco Product Directive (TPD) is used to regulate and supervise the manufacturing, sales, display (product design, packaging, etc.) of products and all tobacco and tobacco-related products (such as electronic cigarette products).
The object of implementation is the EU member states. The Directive requires the member states to convert it into their domestic laws and implement it before May 20, 2016.
The EU TPD certification is to obtain the electronic cigarette product standards and corresponding technical requirements according to the TPD test, and then the inspection and certification authority confirms that the electronic cigarette product has met the corresponding standards and corresponding technical requirements in the EU TPD directive by issuing the certification certificate and certification mark, so as to obtain the license to enter the EU market.
EU TPD detection and registration process for electronic cigarettes
The main contents of the cigarette oil notice shall include:
1. Manufacturer's name and contact information;
2. List of ingredients and content of released substances;
3. Toxicological data;
4. Dose intake information;
5. Product composition and production process description;
6. Statement of safety responsibility;
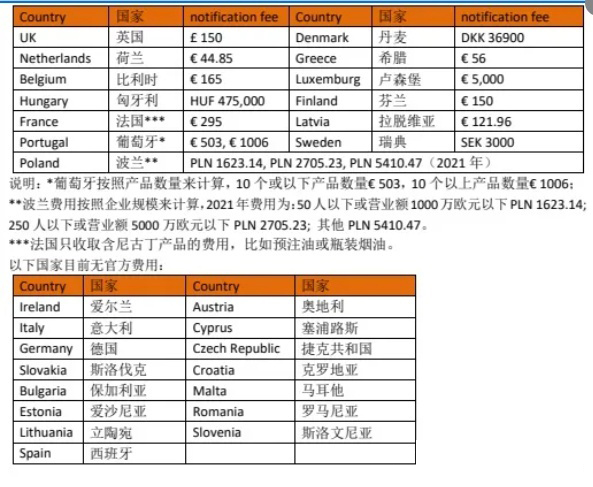
TPD registration fee in EU countries and MHRA registration fee in UK





 Shen Gongwang Security: 44030602006947
Shen Gongwang Security: 44030602006947