The European Commission has issued an authorized regulation to amend the CLP regulation, which stipulates new hazard categories and standards for the classification, labeling, and packaging of substances and mixtures.
It applies to all chemical substances and mixtures placed on the EU market according to REACH. It is also applicable to active substances in biocide products and plant protection products, which are usually given priority in the EU unified classification.
This EU legislation is binding on manufacturers, importers, downstream users, and distributors who place substances on the EU market. Member States will also refer to new hazard categories and standards when proposing unified classification and labeling proposals.
The new hazard categories are:
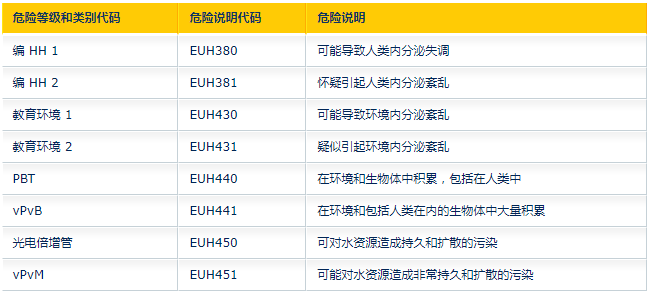
ED HH Categories 1 and 2 (endocrine disruptions to human health)
ED ENV belongs to categories 1 and 2 (environmental endocrine disruptions)
PBT (persistent, bioaccumulative, toxic), vPvB (very persistent, very bioaccumulative)
PMT (persistent, mobile, toxic), vPvM (very persistent, very mobile)
New EU Hazard Statement:
Application date
The new regulations will come into effect from April 20, 2023. Starting from today, member states can propose coordinated classification and labeling (CLH) recommendations for new hazard categories, allowing manufacturers, importers, downstream users, and distributors to self classify their substances and mixtures accordingly.
After the authorization regulations come into effect, there is a transitional period during which manufacturers, importers, downstream users, and distributors have not yet been required to classify their substances or mixtures according to the new hazard categories. During this period, new hazard levels can be voluntarily applied.
At the end of the transition period, all manufacturers, importers, downstream users, and distributors must adopt the new hazard level.
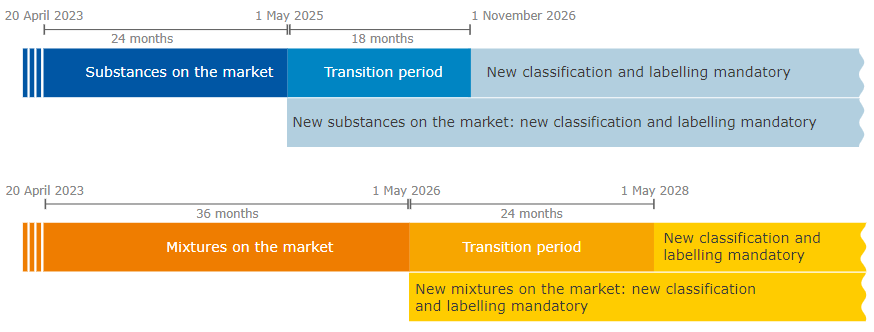
For new substances on the market, companies are required to comply with the new rules starting from May 1, 2025, while substances that have already entered the EU market must comply before November 1, 2026.
A separate transition time applies to the mixture. The new hazard category applies to new mixtures starting from May 1, 2026, and the company must update the classification and labeling of existing mixtures before May 1, 2028.






 Shen Gongwang Security: 44030602006947
Shen Gongwang Security: 44030602006947